Labrador | 21 CFR Part 11 Edition
FDA-compliant electronic records and signatures—without the compliance headache.
Protect data integrity, prove traceability, and move discovery forward.
Why 21 CFR Part 11 Matters—And Why It’s Hard
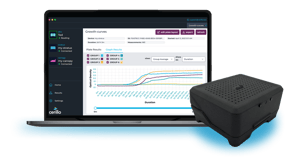
Regulated labs live and die by data integrity. Traditional plate readers and paper logbooks leave gaps—missing audit trails, weak sign-offs, and siloed files. The 21 CFR Part 11 Edition of Labrador bakes in validation, access controls, and real-time audit logging, so you spend less time proving compliance and more time pushing science.

Key Features & Benefits
Every data point is timestamped and water-marked, eliminating manual transcriptions.
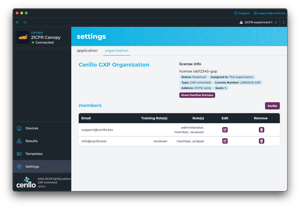
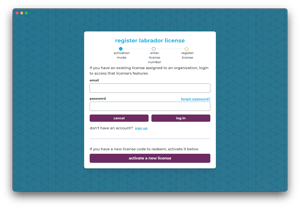
Unique user IDs and granular permissions satisfy FDA system-security requirements.

Automatic, non-editable logs of every change—exportable on demand for inspectors.
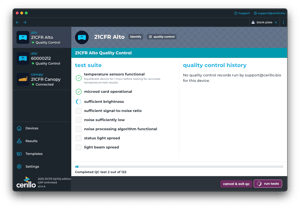
Factory-issued Installation and Operational Qualification files accelerate validation.
Embedded walkthroughs document that staff are trained and certified.
.png?width=300&name=role-based-permissions%20(1).png)
Drop-in upgrade for existing Labrador users with the same intuitive interface.
How It Works
Secure login with e-signature authentication
Run assay—data auto-validated and timestamped
Review audit log; approve or reject with digital signature
Export Part 11-ready PDF for submission
Ready for a No-Risk Pilot?
Experience full compliance on your next run—book a 30-minute guided demo.
Trusted by leading biopharma teams with a 100 % audit-pass record.
info@cerillo.bio
