MBio - Volume 15, Issue 2

Divergent Pseudomonas aeruginosa LpxO Enzymes Perform Site-Specific Lipid A 2-Hydroxylation
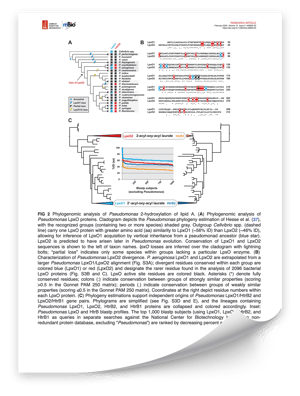
This study explores the unique lipid A 2-hydroxylation mechanisms of divergent Pseudomonas aeruginosa LpxO enzymes. The work delves into the molecular diversity of LpxO enzymes, which are responsible for the hydroxylation of lipid A in bacterial membranes. This process plays a critical role in the virulence and environmental adaptability of Pseudomonas aeruginosa, a bacterium that can cause life-threatening infections, particularly in individuals with weakened immune systems. Understanding these enzymatic variations provides new insights into potential therapeutic interventions.
Access the complete research for:
 Explores the biochemical diversity of Pseudomonas aeruginosa, a critical pathogen in healthcare.
Explores the biochemical diversity of Pseudomonas aeruginosa, a critical pathogen in healthcare.
 Offers insights into lipid A modifications that influence bacterial virulence.
Offers insights into lipid A modifications that influence bacterial virulence.
 Provides a potential target for the development of novel antibacterial therapies.
Provides a potential target for the development of novel antibacterial therapies.
 Demonstrates the application of the Stratus microplate reader in complex biological environments, showcasing its versatility and precision.
Demonstrates the application of the Stratus microplate reader in complex biological environments, showcasing its versatility and precision.
 Key findings contribute to the broader understanding of bacterial adaptation and resistance mechanisms.
Key findings contribute to the broader understanding of bacterial adaptation and resistance mechanisms.
The research utilized Cerillo’s microplate reader system to measure optical density in various microbial growth assays. The Cerillo microplate reader, known for its small footprint and adaptability, was instrumental in providing continuous real-time measurements in challenging environments, such as anaerobic chambers. This allowed researchers to track bacterial growth and enzyme activity under precise conditions, enhancing the accuracy of data collection without manual intervention.

Cerillo's new Alto Microplate Reader provides enhanced precision, greater flexibility, and advanced features, making it an even more powerful tool for cutting-edge microbiological research.
Cerillo thanks Casey E. Hofstaedter and Robert K. Ernst for their highly impactful work and publication.
